ISO 13485 Certification structure and requirements
Is it accurate to say that you are
planning to comprehend the intricate details of ISO 13485 Certification? It is safe to say
that you are the individual devoted to usage of this standard inside your
association? All things considered, without perusing the standard line by line,
enable us to give you a "guide" of ISO 13485 Certification requirement and structure.
The standard
incorporates eight provisos, three of which give direction on the expected
utilization of the standard. The staying five statements give the structure to
what is required of associations devoted to medicinal gadget generation. Along
these lines, this is what to expect so as to accomplish consistence.
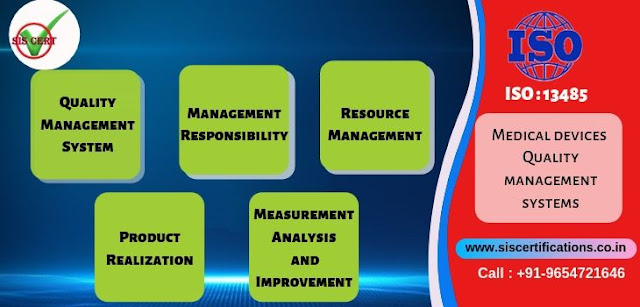
Clause 4 – Quality Management System : QMS (“Intersection”)
Clause-4 targets two quite specific parts of a Quality
Management System: General Requirements and Documentation Requirements.
General Requirements. In assessment of any ISO standard, there are a couple of orderly
necessities that are the main impetus for foundation and usage of a Quality
Management System. The necessities explicit to ISO 13485 Certification incorporate the
accompanying:
1.
Adhere to the
standard.
2.
Document what requirements
to be documented.
3.
Maintain what is expected
of you.
4.
Have composed
methods set up and guarantee the adequacy of the framework that you execute.
5.
Consider the
hazard factors in all exercises.
6.
Introduce steps to
limit the dangers recognized and expect to not cause cataclysmic occasions.
7.
Identify how
things ought to be done to create your restorative gadget and adhere to those
procedures.
8.
Determine
approaches to follow your exercises, right any procedure disappointments or
oversights, and create records to demonstrate every one of the exercises are
being finished.
9.
Determine the
necessities that you are legally bound to, and follow-them!
10. Even when outsourcing work, ensure you
maintain responsibility for that work.
11. Even when redistributing work,
guarantee you keep up obligation regarding that work.
12. Any frameworks utilized in your assembling
procedures ought to be affirmed to guarantee they fill in as planned and don't
adversely influence your procedures.
Documentation Requirements. Most quality frameworks
require a key part, a Quality Manual. Past the Quality Manual, an association
ought to decide the guarantee that they will make to guarantee a situation and
culture that can be steady with putting quality first in all exercises. This
dedication can be caught with a strategy or target explanation. The standard
incorporates unmistakable necessities for the two systems and records, every
one of which must be satisfied:
1.
Medical-device
creation should be accompanied by a file that includes product specifics and
guidance on intended use
2.
Plan for controlling-
documents.
3.
Plan for
controlling-records.
4.
Clause 5 – Management Responsibility (“Highway”)
The Management must
exhibit their responsibility by demonstrating they can be considered
responsible for the tasks inside their association. They need to guarantee that
their center does not hinder from the necessities of the end client, and that
all laws are followed in the assembling procedure. The board has a flat out
obligation to help the quality arrangement, affirm its arrangement with the
laws of the nation of work, and convey the mission to representatives. They
have a duty to design, delegate expert, and impart viably. They are additionally
in charge of an intermittent survey of tasks and improvement inside the
association, known as the Management Review.
Clause 6 – Resource Management (“Roadway”)
Top management has
a responsibility to ensure that the Quality Management System is compliant with
ISO 13485 and adheres to local regulatory requirements. As a requirement within ISO 13485 Certification, top management must ensure that adequate resources are available to
perform the work promised by the organization. Providing resources can refer to
personnel, infrastructure, consumables, equipment, succession planning, and
risk aversion. This can be as specific as controlling the daily workflow to
prevent contaminants or ensuring that operations are seamless in years to come
with an awareness of looming retirements. This commitment from management,
although it may seem minimal, is critical to the organization’s success in
medical device manufacturing and is required according to Clause 6.
Clause 7 – Product
Realization (“Overpass”)
An association
must arrangement for the adventure from conceptualization to execution. This
can incorporate building up a procedure for recording how musings are started,
ideas are confirmed, and items are structured and created, just as how to check
and approve to satisfy the prerequisites for ISO 13485 Certification, Clause 7.
Correspondence is basic for the structure and improvement of the gadget.
The key is to
pursue the procedure from intending to inputs, yields to survey, ahead to
check, trailed by affirmation through approval. Moving thoughts, controlling
the plan, reporting any required changes, and holding all documents
incorporated into the procedure is basic in item acknowledgment. Characterizing
and following supplies, holding basic data related with every item, and
deciding how to confirm these items ought to be obviously archived inside a
system.
Checking each
piece of the procedure includes guaranteeing tidiness, observing portion,
playing out the fundamental administration, and satisfying the necessities
explicit to restorative gadgets. Adequately observing and looking after
hardware, just as guaranteeing that distinguishing proof prerequisites are met
for the gadget itself, are likewise segments of item acknowledgment. In
conclusion, observing the viability of the item as it identifies with
detectability, overseeing client property, and guaranteeing conservation of
item will help accomplish consistence with ISO 13485 Certification.
Clause 8 – Measurement, Analysis and Improvement (“Bridge”)
·
Handling
grievances
·
Reporting events to
administrative experts
·
Undergoing inside
assessments through auditing
·
Continual
procedure and item assessment inside
· Identifying and
controlling items that don't meet the first structure prerequisite (nonconforming
item)
·
Analyzing
information produced and ceaselessly improving the procedure
Concluding via “Thoroughfare”
The
"guide" plot above offers you a chance to comprehend both the
structure and the prerequisites for consistence with ISO 13485 Certification. With a
comprehension of the five basic provisos, we urge you to investigate the way
for implementation.










Comments
Post a Comment